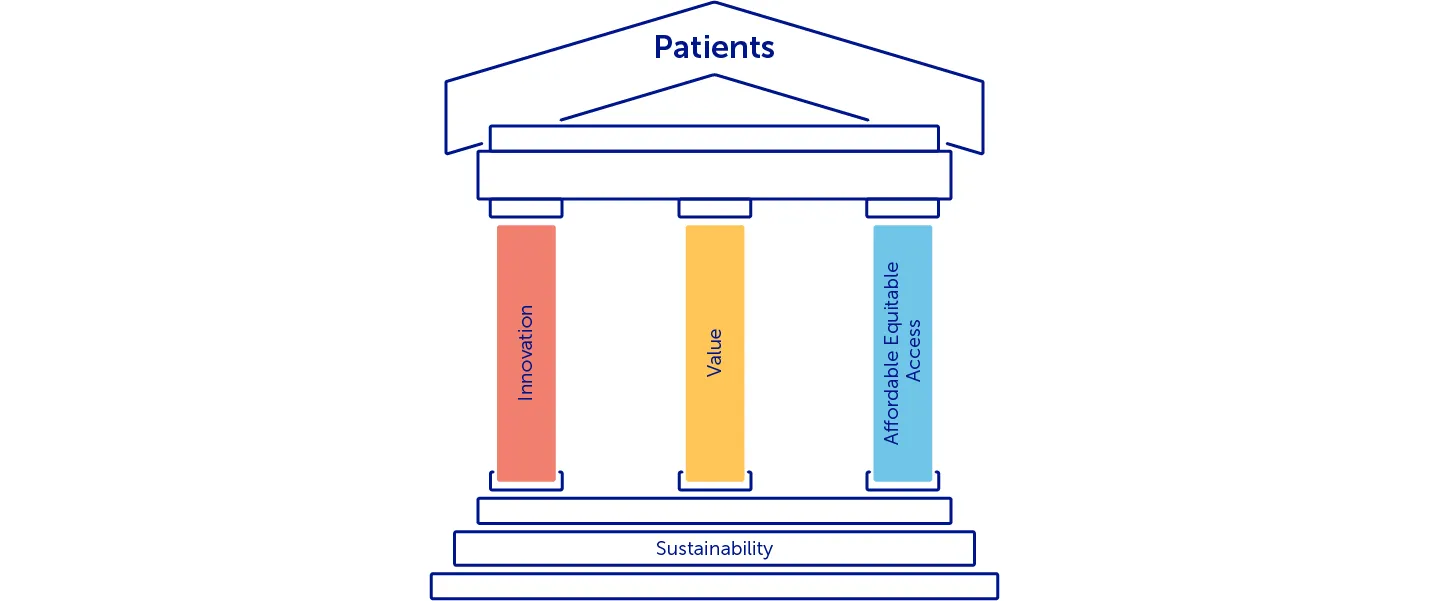
This approach guides our engagement and partnerships in the healthcare ecosystem and in public policy. At UCB, patients are at the center of our approach to public policy. Therefore, we focus on promoting innovation that provides solutions for unmet clinical needs, ensuring that the value of medicines is fairly recognized, and that patients have equitable, affordable access to the care they need.
At UCB, sustainability means we recognize the essential link between human health and the health of the planet, while also focusing on ensuring that patients who need access to our medicines in a way that is viable for patients, society, and UCB. We strive to make a meaningful difference now and into the future - by focusing our policy work not only on what will make an impact in the short-term, but also over the long-term, keeping both current and future generations in mind. We do not advocate for policies that would come at the expense of continued innovation and inclusion, or transparency and integrity.
UCB recognizes that we are part of a healthcare ecosystem system, and a society that are continually undergoing change. The critical importance of a sustainable healthcare system has rarely been more apparent as a result of not only a global pandemic, but also the recognition of social inequalities and the dramatic impact of climate change. Our commitment to patient value means that our approach to public policy must recognize and adapt to these changes and that our advocacy continues its focus on delivering solutions which meet both patients’ and society's evolving needs.
UCB also recognizes the healthcare ecosystem is comprised of multiple stakeholders with unique perspectives. Each of those stakeholders has a unique role to play in working to improve individual patients’ health, as well as achieving public health priorities. Considering this dynamic, UCB continually encourages stakeholders to work together to achieve these dual goals, including through strategic partnerships to find ways to amplify individual contributions. Through our actions, engagement, and partnerships, we are dedicated to the continued evolution of an equitable policy environment that recognizes and rewards innovation, encourages value-based care, and promotes affordable access to medicines for patients.