The AIM Movement provides support, information and a place to share stories about motherhood in women with chronic inflammatory diseases such as rheumatoid arthritis (RA), psoriatic arthritis (PsA), ankylosing spondylitis (AS), and Crohn’s disease (CD).
Atlanta, GA – 11 July 2018 – UCB, a global biopharmaceutical company, today announced the launch of the Autoimmune Motherhood (AIM) Movement to support women living with chronic inflammatory or autoimmune diseases such as rheumatoid arthritis (RA), psoriatic arthritis (PsA), ankylosing spondylitis (AS), and Crohn’s disease (CD), as they attempt to balance disease management with concerns and considerations related to family planning, pregnancy and breastfeeding. The movement will rally these and other women by providing information and creating an interactive online community where they can connect with and empower one another by sharing their stories and experiences.
“Women with RA, PsA, AS and CD should work with their physicians to maintain a fully-informed disease management plan throughout their lives, and this does not change during important times such as during pregnancy or breastfeeding. In fact, it becomes more important for both mother and child, since data shows that active CD and RA can lead to adverse pregnancy outcomes," said Dr. Grace Wright, specialist in Rheumatology at New York University (NYU) Langone Medical Center. "For too long, many of these women have had inadequate information about appropriate care and the new AIM Movement will help them address their concerns, learn from others and make the best decisions for themselves and their families.”
The new AIM Patient Survey, a global survey of 1,052 women (457 U.S. respondents, 561 in major European nations, and 34 in Japan) of childbearing age (18-45) living with chronic inflammatory diseases like RA, PsA, AS, and CD, suggests that lack of information about their situation impedes the ability of these women to make informed decisions about disease management and motherhood:i
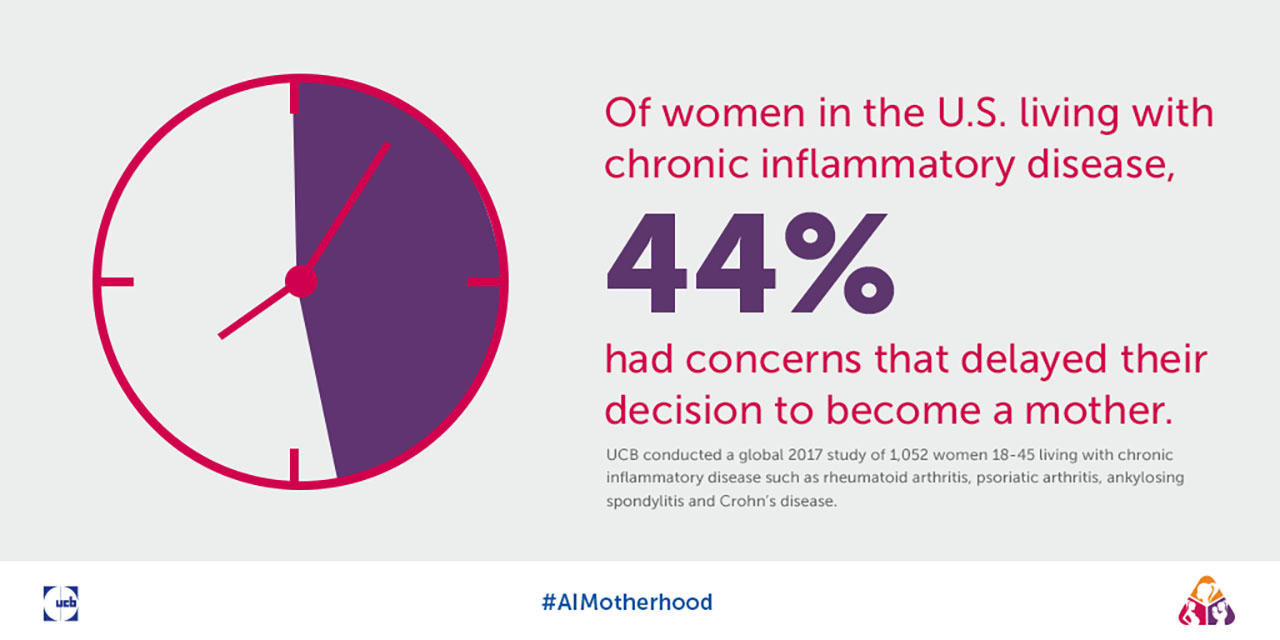
- Almost half (44%) of surveyed women in the U.S. had concerns serious enough to lead them to delay their plans to become pregnant.
- Over a third (36%) of U.S. women decided to discontinue their treatment while planning their pregnancy or at the start of their pregnancy.ii
- Almost two out of three (61%) of U.S. women felt they could not combine treatment and breastfeeding. ii
- Yet, while many express concern, only 41% of women surveyed in the U.S. consulted a healthcare professional before becoming pregnant, suggesting the need for women to become more engaged in treatment and pregnancy planning earlier.ii
“When I first started thinking about having a family, I felt alone, unsure where to turn for guidance on how to balance disease management and pregnancy. I had always assumed that becoming pregnant would mean I would have to stop my treatment, so I thought I had to choose between concerns for my baby and my own health,” said Rosanna, a mother of two living with rheumatoid arthritis. “Once I realized that I’m not the only woman in this situation, I felt more confident talking to my doctor about my hesitations and finding a treatment plan that I can feel good about.”
The AIM Movement will mobilize an awareness campaign and resource community to help address some of the concerns and challenges experienced by these women throughout their reproductive health journey. It will offer a space for patients to connect with their peers to empower one another, equip them with disease management information and support them as they engage their physician in proactive discussions about family planning.
“Many women living with chronic inflammatory diseases such as RA, PsA, AS and CD feel unprepared and see no choice but to change their plans for a family or shortchange their own disease management plan as they are planning for families, when maintenance of disease control is important,” said Todd Edwards, Head of U.S. Immunology and Vice President at UCB. “We are strongly committed to empowering women through the UCB AIM Movement to have the fullest, most positive experience possible during these special years, regardless of their disease status. This means knowledge and access to appropriate care and the opportunity to be part of a supportive community when it is needed most.”
The AIM Movement launches today across the U.S. Learn more about the AIM Movement at www.AIMotherhood.com and share your story by visiting the website or posting on social media using the hashtag #AIMotherhood.
Click to Tweet: For women with #autoimmune diseases such as RA, PsA, AS and CD, find support at the intersection of womanhood, disease management and family planning. Be part of the #AIMotherhood movement: www.AIMotherhood.com
About the AIM Patient Survey
In 2017, UCB conducted the AIM Patient Survey, a global study of 1,052 women 18-45 living with chronic inflammatory disease such as rheumatoid arthritis (RA), psoriatic arthritis (PsA), ankylosing spondylitis (AS), and Crohn’s disease (CD), who were pregnant or had been pregnant in the past 2-5 years at the time of the survey. The goals of the survey were to identify the needs and concerns of women of childbearing age and gain better insight into the treatment landscape for this underserved patient population. The survey was administered online by InSites Consulting between July and October, 2017.
For further information:
Corporate Communications
France Nivelle,
Global Communications, UCB
T +32.2.559.9178, france.nivelle@ucb.com
Laurent Schots,
Media Relations, UCB
T +32.2.559.92.64, laurent.schots@ucb.com
Investor Relations
Antje Witte,
Investor Relations, UCB
T +32.2.559.94.14, antje.witte@ucb.com
Brand Communications
Andrea Levin Christopher,
Immunology Communications, UCB
T +1.404.483.7329
andrea.christopher@ucb.com
About UCB
UCB, Brussels, Belgium (www.ucb.com) is a global biopharmaceutical company focused on the discovery and development of innovative medicines and solutions to transform the lives of people living with severe diseases of the immune system or of the central nervous system. With more than 7,500 people in approximately 40 countries, the company generated revenue of € 4.2 billion in 2016. UCB is listed on Euronext Brussels (symbol: UCB). Follow us on Twitter: @UCB_news
Forward looking statements
This press release contains forward-looking statements based on current plans, estimates and beliefs of management. All statements, other than statements of historical fact, are statements that could be deemed forward-looking statements, including estimates of revenues, operating margins, capital expenditures, cash, other financial information, expected legal, political, regulatory or clinical results and other such estimates and results. By their nature, such forward-looking statements are not guarantees of future performance and are subject to risks, uncertainties and assumptions which could cause actual results to differ materially from those that may be implied by such forward-looking statements contained in this press release. Important factors that could result in such differences include: changes in general economic, business and competitive conditions, the inability to obtain necessary regulatory approvals or to obtain them on acceptable terms, costs associated with research and development, changes in the prospects for products in the pipeline or under development by UCB, effects of future judicial decisions or governmental investigations, product liability claims, challenges to patent protection for products or product candidates, changes in laws or regulations, exchange rate fluctuations, changes or uncertainties in tax laws or the administration of such laws and hiring and retention of its employees. UCB is providing this information as of the date of this press release and expressly disclaims any duty to update any information contained in this press release, either to confirm the actual results or to report a change in its expectations.
There is no guarantee that new product candidates in the pipeline will progress to product approval or that new indications for existing products will be developed and approved. Products or potential products which are the subject of partnerships, joint ventures or licensing collaborations may be subject to differences between the partners. Also, UCB or others could discover safety, side effects or manufacturing problems with its products after they are marketed. Moreover, sales may be impacted by international and domestic trends toward managed care and health care cost containment and the reimbursement policies imposed by third-party payers as well as legislation affecting biopharmaceutical pricing and reimbursement.
References:
i N. Thyssen, M. Geens, C. Jauquet, E. Van den Eeckhaut. “WoCBA Patient Survey Key Topline Findings”. Questionnaire. 16 Nov. 2017.
ii N. Thyssen, M. Geens, C. Jauquet, E. Van den Eeckhaut. “WoCBA Patient Survey Raw Data”. Questionnaire. 16 Nov. 2017.
---
USP-DSRH0218-0006
Choose Country
- Global Site – English
- Australia – English
- België – Engels
- Belgique – Anglais
- Brasil – Português
- България – Български
- Canada – English
- Canada – Français
- 中国 – 中文
- Česká Republika – Angličtina
- Danmark – Engelsk
- Deutschland – Deutsch
- France – Français
- España – Español
- Ελλάδα – Ελληνικά
- India – English
- Ireland – English
- Italia – Inglese
- 日本 – 日本語
- Казахстан – ағылшын тілі
- 한국 – 한국어
- Luxembourg – Anglais
- Luxemburg – Engels
- Magyarország – Angol
- México & Latinoamérica – Español
- Nederland – Engels
- New Zeeland – English
- Norge – Engelsk
- Österreich – Deutsch
- Polska – Polski
- Portugal – Inglês
- România – Engleză
- Россия – Русский
- Slovensko – Anglický
- Suomi – Englanti
- Sverige – Engelska
- Schweiz – Deutsch
- Suisse – Français
- Türkiye – Türkçe
- Україна – Англійська
- United Kingdom – English
- U.S.A. – English